Why Buffer Solution Is Used in Edta Titration
Download the pH 10 ammonia buffer solution preparation file. A all reactions between metal ions and EDTA are pH dependent and for divalent ions solutions must be kept basic and buffered for.

The Phosphorus P Status Of 535 Surface Soils From All States Of Australia Was Assessed Using The Foll Soil Classification Soil Testing University Of Adelaide
Ad Titration applications can be tricky.
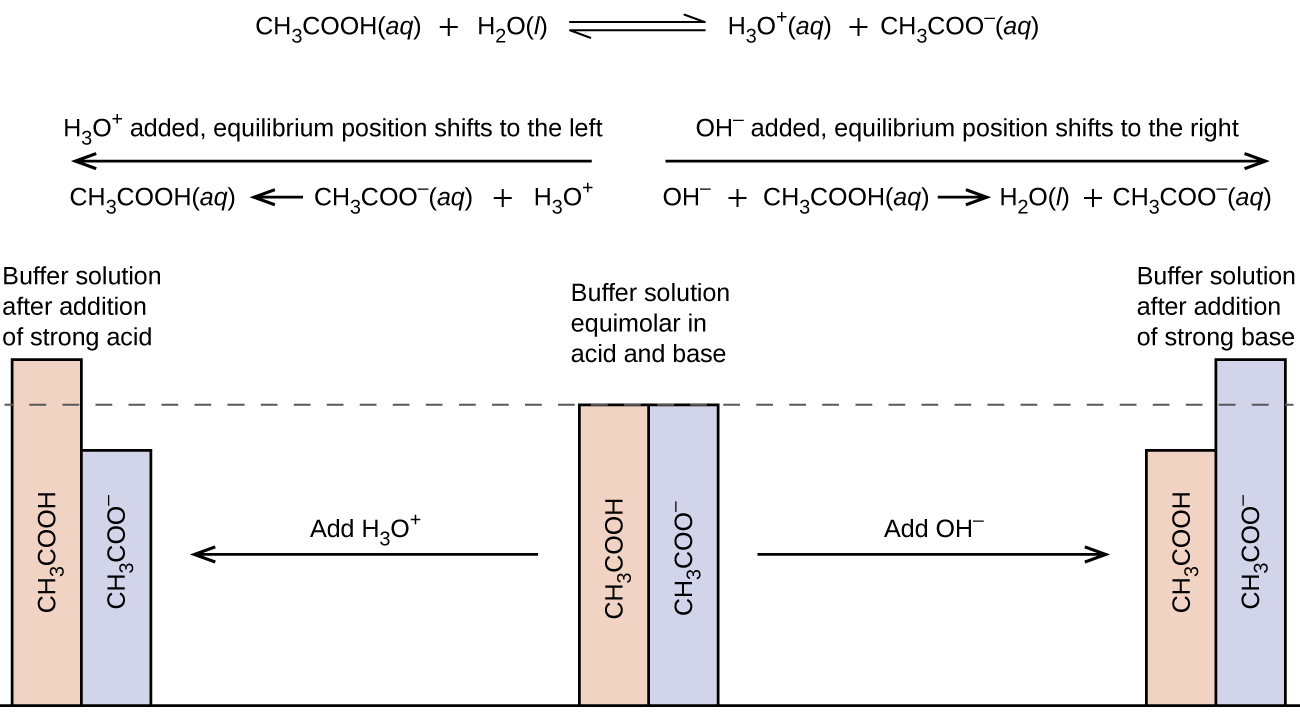
. Change in pH may lead to the improper reaction between Metal ion and EDTA. Answer 1 of 6. After opening the file.
Because all reactions between metal ions and EDTAare pH dependent. The principal reason that EDTA is utilized so broadly in the normalization of metal cation solution is that the development steady for most metal cation-EDTA complexes is high. It is necessary to keep the pH at about 10 for two reasons 1.
But I assume the addition of ceCuEDTA2- solution to your unknown cePb sample is to get a sharp end-point example of Replacement or Substitution Titration. Always remember that a buffer is added to the solution that undergoes titration in a specific pH range. It is necessary to keep the pH at about 10 for two reasons.
3 13 13 19. Both the metal ions and EDTA are pH- dependent. Why do we use buffer solution in EDTA titration.
The important indicator used for non-aqueous titration are follow-1. EDTAis commonly useda complexing agent which make a complex with metal ions. Open it with the free trial version of the buffer calculator.
The eriochrome black T. To prepare the recipe for a needed volume of the solution use ChemBuddy Buffer Maker program. It has four carboxyl groups and two amine groups that can act as electron pair donors Lewis bases.
While this is not our intent when standardizing EDTA solution at this high pH it is possible to titrate calcium in the presence of magnesium as the latter precipitates in the form of Mg OH 2. Reaction taking place during titration is. EDTA is often used as the disodium salt Na2H2Y.
We have to know that the reason why the buffer is used in EDTA titration is because it avoids the pH change. It is necessary to keep the pH at about. The Buffer solution is used to resist the change in pH.
Which indicator is used in non aqueous titration. Change in pH may lead to the improper reaction between Metal ion and EDTA. Ca 2 EDTA 4- CaEDTA 2-.
Good Titration Practice can be tricky. EDTA often written as H4Y is a common ligand in complexometric titrations. Our AutoTitrators come with built-in expertise.
The buffer solution is used in EDTA titration because it avoids the change in pH so that reactions take place between metal ions and EDTA. Concentrated pH 10 ammonia buffer. The Buffer solution is usedto resist the change in pH.
PH 10 buffer is used in EDTA titration because in EDTA Y4- is predominant and we want Y4- to react with the metal ions that are present in the. Carrying out the reaction in a basic buffer solution removes the H as it is formed. Since the extra metal ion added ceCu2 has already complexed.
PH 10 buffer is used in EDTA titration because in EDTA Y4- is predominant and we want Y4- to react with the metal ions that are present in the titration solution. Stay tuned with BYJUS to learn more about other concepts such as titration. Im not an expert on coordination chemistry and in particularly metal-indicator complexes in EDTA titrations for heavy metals.
Improve your accuracy with our Automated Titrators. This is a concentrated buffer of high ionic strength added in small amount to titrated samples. To maintain high alkaline medium ammonia buffer is added to EDTA in analysis of hard water.
Buffer maintains the pH of the solution through out the reaction. After opening the file. Why buffer solution is used in EDTA titration.
This can be achieved by using a pH 10 buffer. Titration is done in about 01 sodium hydroxide solution pH around 13. Was this answer helpful.
All reaction between metal ions and EDTA are pH dependent and for divalent ions solution must be kept basic and buffered for reaction to go to completion. Crystal voilet- It is used as 05 solution in glacial acetic acid it gives voilet colour in basic medium and yellowish green in acidic medium. It is most widely use for the titration of pyridine with prechloride acid.

Determining Total Hardness Of Supply Water By Edta Buffer Solution Conical Flask Flask

Determining Total Hardness Of Supply Water By Edta Buffer Solution Conical Flask Flask
What Does It Mean When A Titration Curve Doesn T Have Buffer Quora

Why Buffer Solution Is Used In Edta Titration

Solved A For Edta Titration Why Is It Important To Buffer Chegg Com

Why Use A Ph 10 Buffer In Edta Titration Quora

A Practical Guide To The Preparation And Use Of Metal Ion Buffered Systems For Physiological Research Neumaier 2018 Acta Physiologica Wiley Online Library

Polyprotic Acid Base Part 2 Buffers Youtube

Why Ammonia Buffer Is Used In Edta Titration
What Does It Mean When A Titration Curve Doesn T Have Buffer Quora
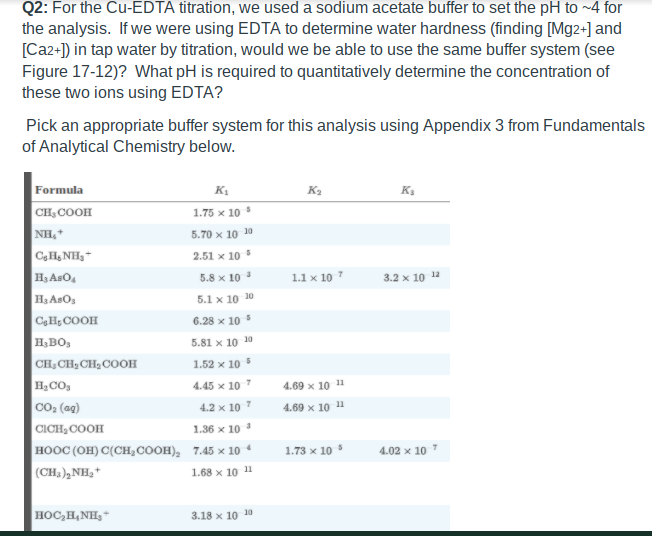
Solved Q2 For The Cu Edta Titration We Used A Sodium Chegg Com

Na Chomu Polyagaye Efekt Krioskopiyi Physical Properties Physics Science Chemistry

Hardness Of Water By Edta Titration

Why Use A Ph 10 Buffer In Edta Titration Quora

Kent Marine Concentrated Liquid Calcium Marine Aquarium Calcium Food Animals


Comments
Post a Comment